Washing In Hard Water
Water described as “hard” is high in dissolved minerals, specifically calcium and magnesium. All water contains calcium; the amount depend on what kind of crystalline rock it runs over.
Hard water is not a health risk , it is actually a very valued and our easiest source of calcium. For our health it is better to have hard water. The recommended water hardness for human consumation is between 1,1 - 5 mmol/l, thats 6,16 – 28 oN (1mmol/l = 5,6 oN).
Hard water is healty for people but not for our washing or pipes. It is a nuisance because of mineral buildup on fixtures and poor soap and/or detergent performance. The degree of hardness becomes greater as the calcium and magnesium content increases.
How to fight hard water in household?
* Add few drops of lemon juice to your drink water.
* Reduce the temperature of your boiler – As the water temperature increases, the more mineral deposits will appear in your dishwasher, water tank and pipes. By reducing the heat of your boiler to about 55ºC, you will have enough hot water for your shower and you will reduce the amount of mineral build-up in your pipes and tanks.
* You can use white vinegar by using the dishwasher dispenser or placing a cup of vinegar on the dishwasher rack. Boil some white vinegar in your kettle as a useful way of removing hard water deposits. Add some vinegar to your rinse cycle. (Vinegar is indeed good for clothes, but can attack plastic and metal parts of the machine.)
Washing in hard water:
There is an old (german, still used for detergents) hard water classification in oN and a new hard water clasiffication used mostly by water companies in mmol (1 mmol/l = 5,6 oN). On detergent labels you will find mostly 4 degrees of water hardness:
0 - 7 °d soft
7 - 14 °d slightly hard
14 - 21 °d hard
over 21 °d very hard
Clothes washed in hard water often look dingy and feel harsh and scratchy. The hardness minerals combine with some soils to form insoluble salts, making them difficult to remove. Soil on clothes can introduce even more hardness minerals into the wash water. Continuous laundering in hard water can damage fibers and shorten the life of clothes by up to 40 percent.
* There are washing powders and liquids available for a wide range of water hardness. Make sure you choose the correct detergent for your area; you may also need to use slightly more detergent than the manufacturers recommended amount to compensate for the hard water. In many cases the manufacturer will give specific instructions on how to use the product in hard water areas, look out for these labels on your product. Add the recommended amount of detergent for hard water or more, but to be sure to get it all out add enough water and vinegear to your last rinse.
* You can try an extra water softener. Water Softeners work by ion exchange, so sodium replaces the calcium and/or magnesium in the water, for example Sodasan Water Softener (sodium silicate.
* Think about installing a magnetic water conditioner. It's much cheaper and more effective way to deal with the hard water. Water Conditioners work by altering the crystallisation behaviour of Calcium and Magnesium ions such that they loose their ability to cause adhesive scale:
- Safe money for more detergents.
- Permenantly installed in plumbing system to alter calcium ions so they cannot cause limescale.
- Output water is fit for drinking, calcium retained, good for diet.
- Lengthens lifespan of clothes.
- Low running cost.
|
Overall Water Hardness 1) |
Hardness Degree [°dH] |
Hardness Degree [mmol/l] |
|---|---|---|
|
water very soft |
0 to 4 |
0 to 0,72 |
|
water soft |
4 to 8 |
0,72 to 1,43 |
|
water slightly hard |
8 to 12 |
1,43 to 2,14 |
|
water moderately hard |
12 to 18 |
2,14 to 3,21 |
|
water hard |
18 to 30 |
3,21 to 5,35 |
|
water very hard |
> 30 |
> 5,35 |
1) With reference to: Hydrochemie, Pavel Pitter, 4. edition, VŠCHT in Prague, 2009
[°dH] = german classification
Conversion
1 mmol/l = 5,6 °dH
1 °dH = 0,1783 mmol/l .
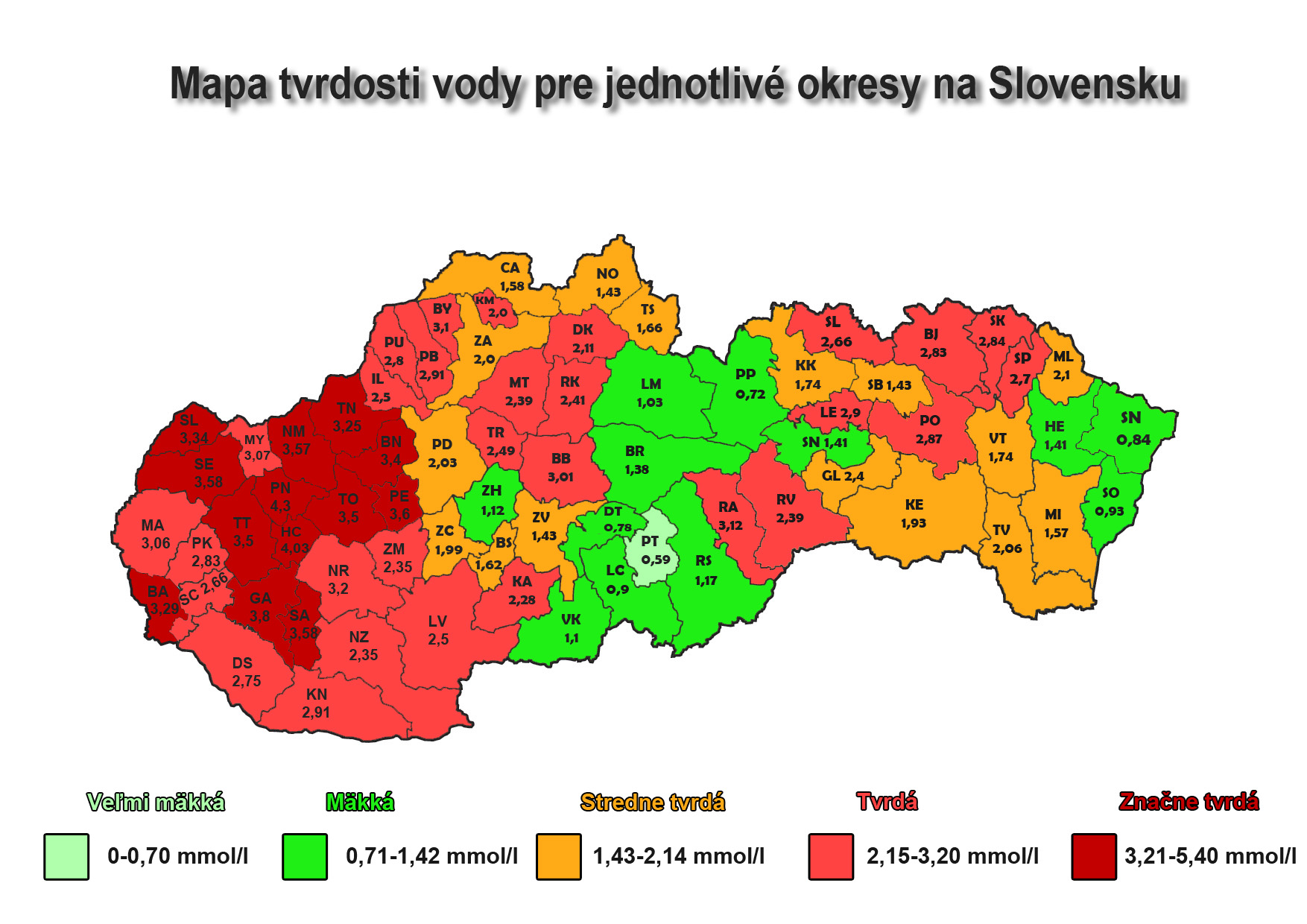
Water Hardness in some regions:
west Slovakia
north Slovakia
Bratislava
United Kingdom
France
USA